GMP Storage Market to Grow at 5.6% CAGR Through 2033 | Persistence Market Research
GMP storage market supports compliant storage of biologics, vaccines, and cell therapies, driven by innovation, outsourcing, and manufacturing growth.
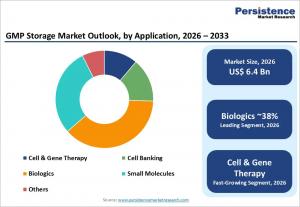
LONDON, UNITED KINGDOM, January 28, 2026 /EINPresswire.com/ -- The GMP storage market is gaining strategic importance as pharmaceutical and biopharmaceutical companies expand research pipelines and scale advanced therapies. The global GMP storage market size is estimated to grow from US$ 6.4 billion in 2026 to US$ 9.4 billion by 2033, registering a CAGR of 5.6% during the forecast period. GMP storage ensures validated, controlled environments for temperature sensitive materials such as vaccines, biologics, blood products, and cell based therapies, reducing risks of contamination, degradation, and regulatory noncompliance. As development timelines shorten and product complexity rises, reliable storage has become a core enabler of efficient research, clinical trials, and commercialization.
Download Your Free Sample & Explore Key Insights: https://www.persistencemarketresearch.com/samples/33113
Market Drivers
Technological adoption is a primary driver transforming the GMP storage landscape. Biomedical refrigerators, ultra low temperature freezers, and cryogenic systems increasingly incorporate IoT enabled monitoring, remote diagnostics, automated alarms, and digital data logging. These technologies reduce temperature deviations, enhance audit readiness, and improve energy efficiency. During 2024 and 2025, adoption of eco friendly refrigeration systems accelerated across pharmaceutical, academic, and clinical settings, reflecting demand for precision and sustainability. Major players such as Thermo Fisher Scientific, Eppendorf, and PHC Corporation continue investing in smart cold chain innovations, launching energy efficient ultra low temperature freezers and intelligent control platforms. These developments support scalable GMP infrastructure for CDMOs and biopharma companies managing growing volumes of biologics and advanced therapies.
Market Restraints
Despite strong growth potential, the market faces pressure to deliver cost effective storage solutions. Establishing GMP compliant infrastructure requires heavy capital investment, driven by stringent validation, qualification, and monitoring requirements. Energy consumption represents a major operational expense, particularly for ultra low temperature and cryogenic systems requiring uninterrupted power supply. Advanced features such as real time monitoring, cloud based data management, and frequent inspections further increase costs. For some cold storage operators, electricity expenses account for a significant share of operating margins. Balancing regulatory compliance, service quality, and affordability remains a critical challenge, particularly for small manufacturers and emerging biotech firms.
Get Custom Insights Designed for Your Business: https://www.persistencemarketresearch.com/request-customization/33113
Market Opportunities
Significant opportunities lie in digitalized, energy efficient, and automated GMP storage solutions. Automated systems with continuous monitoring, predictive maintenance, and remote access reduce manual intervention and minimize product loss. These capabilities are especially valuable for CDMOs and research organizations handling high sample volumes. Another major opportunity stems from rapid growth in cell and gene therapy manufacturing and the expansion of biomanufacturing hubs in Asia Pacific. Countries including China, Japan, India, and Singapore are investing heavily in GMP facilities, innovation parks, and regenerative medicine programs. This expansion is driving demand for cryogenic storage, ultra low temperature freezers, and validated biobanking infrastructure aligned with international standards.
Category Analysis
By products and services, GMP storage products dominate the market, accounting for approximately eighty three percent of revenue in 2025. Biomedical refrigerators, standard freezers, ultra low temperature units, and cryogenic systems form the backbone of GMP compliant storage. These products are essential for maintaining validated conditions for biologics, vaccines, plasma derivatives, and cell therapies. Although storage services are growing due to outsourcing trends, capital investment in high performance equipment remains indispensable. By application, biologics storage represents the largest segment, holding about thirty eight percent market share in 2025. Monoclonal antibodies, recombinant proteins, and complex vaccines require strict temperature control throughout manufacturing and distribution, sustaining strong demand for GMP validated storage systems.
Regional Insights
North America leads the global GMP storage market, driven by strong biologics production, high research spending, and strict regulatory enforcement. The United States hosts a dense network of biopharma companies, CDMOs, and cell therapy developers, increasing demand for cryogenic and monitored storage. Asia Pacific is the fastest growing region, supported by government incentives, expanding biomanufacturing capacity, and alignment with global GMP standards. Rising participation in multinational clinical trials and lower operating costs are accelerating deployment of GMP storage infrastructure across the region.
Checkout Now & Download Complete Market Report: https://www.persistencemarketresearch.com/checkout/33113
Competitive Landscape
The GMP storage market is moderately fragmented, with competition among global equipment manufacturers, cryogenic specialists, and regional service providers. Companies compete on energy efficiency, temperature stability, digital integration, and regulatory expertise. Recent developments include capacity expansions, partnerships with cell and gene therapy developers, and investment in smart connected storage platforms, positioning GMP storage as a critical pillar of the global life sciences supply chain.
Market Segmentation
By Product Type
GMP Storage Products
Refrigerators and Freezers
Cryogenic Storage
GMP Storage Services
By Application
Cell & Gene Therapy
Cell Banking
Biologics
Small Molecules
Others
By End-user
Biopharmaceutical Companies
Contract Manufacturing Organizations
Contract Research Organizations
Research & Academic Institutes
By Region
North America
Europe
East Asia
South Asia and Oceania
Latin America
Middle East and Africa
Read Related Reports:
Flavors into Over-the-Counter (OTC) Pharmaceuticals Market: The global flavors in the over-the-counter (OTC) pharmaceuticals market size is valued at US$2.3 Bn in 2025 to US$3.7 Bn by 2032.
Fibroblast Growth Factors Market: Global fibroblast growth factors market to reach US$462.0 Mn by 2032, expanding at 6.3% CAGR as demand increases for targeted therapies and regenerative treatments.
Persistence Market Research
Persistence Market Research Pvt Ltd
+1 646-878-6329
email us here
Visit us on social media:
LinkedIn
Instagram
Facebook
YouTube
X
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.